|
|||
| ろ過 | FILTRATION | ||
| ろ過処理は水から浮遊物を取り除くために実施する単純な機械的処理です。多くの場合、ろ過処理の前後に化学物理的または生物学的処理を実施します。様々な用途に対し、ろ過速度や濾過床の構成を変えることで、ろ過処理能力を向上させることができます。 主な用途: ・川、泉、井戸、工業水および都市水のろ過 ・工業排水からの汚染物質の吸収 ・限外ろ過、逆浸透、樹脂工場の前処理 ・鉄およびマンガン除去処理
|
 |
Filtration process is a simple mechanical treatment applied to water for the suspended solid removal. Filtration is often preceded or followed by chemical-physical or biological treatments. The choice of filtration speed and filter bed composition establish the efficiency of the filtration process for the different applications. Main applications: River, spring, well, industrial and civil water filtration. Absorption of pollutant substances in industrial effluents. Pre-treatment for ultrafiltration, reverse osmosis, resin plant. Iron and manganese removal process. |
|
 |
|||
| 軟水化 | SOFTENING | ||
| 水の硬度は、特定の塩類が原因で発生します。硬度を高める主なイオンは、カルシウム(Ca2+)、マグネシウム(Mg2+)、重炭酸塩(HCO3-)です。 これらのイオンやミネラルは通常、パイプや飲料水用機器および水処理装置内に溜まる水垢を引き起こすものとして見られます。 軟水化装置は硬水の浄水化および石灰カスの除去を行うことができます。 飲料水、ビールやソーダに含まれる水の生成だけでなく、冷却水やボイラー給水を準備する際、水の硬度は非常に重要です。軟水化装置は、多重正電荷を用いてイオンを除去するために作られた特殊な装置となります。 軟水化装置は、塩化ナトリウム(NaCl)で自動的に再生させることができます。 |
 |
Hardness in water is caused by certain salts. The main ions causing hardness are Calcium (Ca2+), Magnesium (Mg2+) and Bicarbonate (HCO3-). These ions or minerals are normally addressed as scale in the water causing scaling of pipes and equipment in drinking water and process water systems. |
|
 |
|||
| ウルトラろ過 | ULTRAFILTRATION | ||
|
メンブレン(膜)ウルトラろ過は、表層水処理、調整と殺菌、市水および工業用排水処理のための便利で信頼性の高い分離処理を行うことのできる装置です。 |
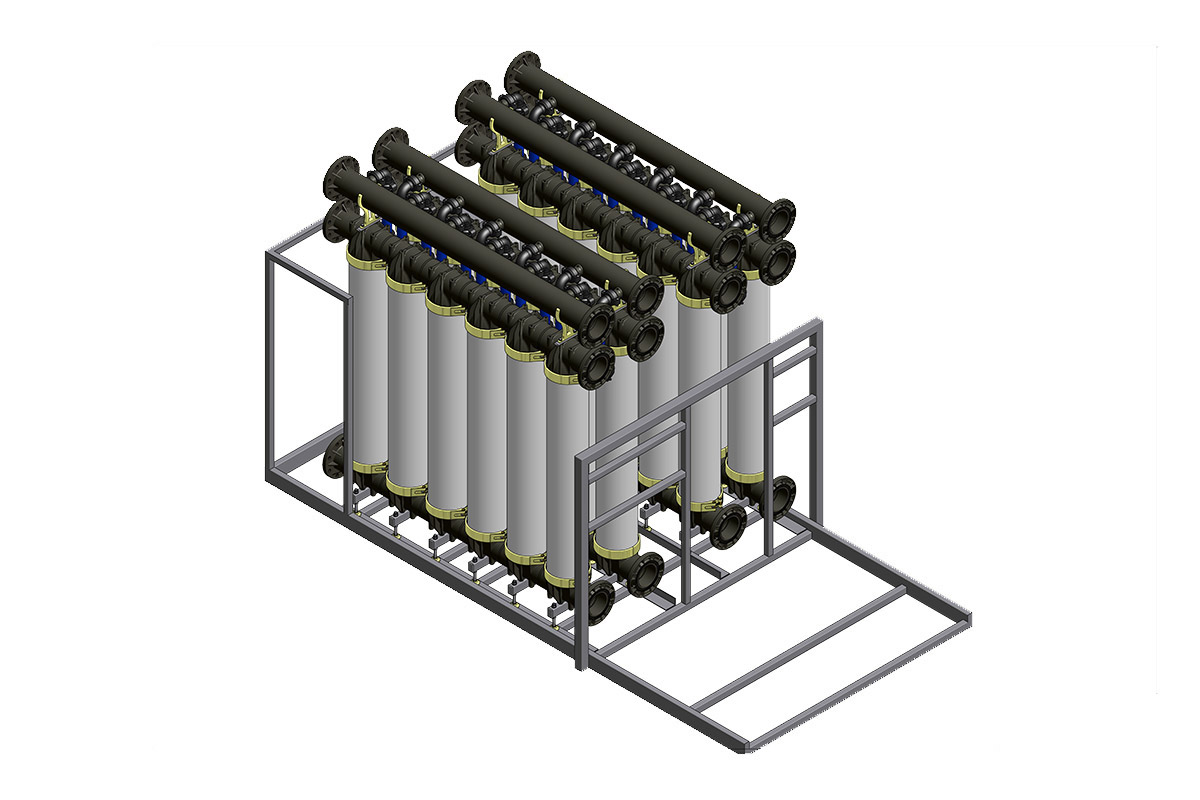 |
Membrane ultrafiltration represents a convenient and reliable technology of separation for treatments of surface water, conditioning and sterilization, civil and industrial sewage. Advantages: Flexibility of operation in the presence of turbidity and suspended solid peaks. Modularity resulting in a fast replacement of the membranes. Modularity of the system with a minimum request for additional spaces, whereas a capacity increase is required. Efficient and effective water treatment generally requires a combination of different methods and technologies. This combination depends on the intended purpose of the cleaned water (e.g. drinking water, industrial process water for power plants, etc.) as well as on the quality and degree of contamination of the original water. Thanks to its unique advantages, ultrafiltration can play a central role in this mix of physical, chemical and mechanical processing methods. |
|
 |
|||
| 逆浸透 | REVERSE OSMOSIS | ||
|
逆浸透は食塩水に浸水性膜(溶解固形物は不浸透)を接触させ、溶液の浸透圧より大きい圧力をかけた際に発生するプロセスです。元の溶液よりも塩分濃度の低い溶液(浸透性)は膜を通過しますが、塩分濃度の高い溶液(濃縮など)は、膜の外側に残ります。 |
 |
Reverse Osmosis is the process which occurs when a saline solution is put in contact with a water permeable membrane (but impermeable to the dissolved solids) at a pressure higher than the osmotic pressure of the solution. A solution (permeate) with a saline content lower than the original solution will pass through the membrane, while a salt rich solution (concentrate) will be determined outside the membrane. The Reverse Osmosis process is the reverse of the “direct” osmosis process which occurs naturally in the absence of pressure external to the system. When two solutions of different concentration and content in two different containers are put in contact through a semi-permeable membrane, there is a flow from the less concentrated solution to the more concentrated solution, leading to a reduction of the concentration in the latter. At the end of the flow, an inequality between the two solutions is evident which represents the value of the osmotic pressure of the more concentrated solution. The Reverse Osmosis process can occur only by applying a pressure higher than the osmotic pressure to the more concentrated solution, which allows the passage of the solvent from the concentrated solution to the less concentrated solution. Higher the value of the pressure applied, higher the permeate flow. |
|
 |
|||
| イオン交換 | ION EXCHANGE | ||
|
イオン交換樹脂は不溶性の粒状物質で、交換可能な分子構造酸または基本ラジカルに含まれます。ラジカルに付いた正および負イオンが接触させた溶液内の同じ符号のイオンと交換されます。イオン交換は、以下の状況を発生させることなく実行することが可能です。 |
 |
Ion Exchange resins are insoluble granular substances which have in their molecular structure acid or basic radicals that can be exchanged. The positive or negative ions fixed on these radicals are replaced by ions of the same sign in the liquid solution in contact with them. The ion exchange is complete without: – Deterioration or solubilisation – Changing the total number of ions in the liquid before the exchange Today the ion exchange substances are used almost exclusively under the name of resins. There are two categories of resins: the resins of gel type and those of macroporous or loosely cross-linked type. Their basic structure is the same: the macromolecular structure is obtained in both cases by co-polymerization. The difference between them lies in their porosity. – Gel type resins have a natural porosity limited to intermolecular distances. It is a microporous type structure. – Macroporous type resins have an additional artificial porosity which is obtained by adding a substance designed for this purpose. The exchanger is known as monofunctional if there is only one variety of radicals and it is called polyfunctional if the molecule contains various type of radicals. |
|
 |
|||
| 電気脱イオン | ELECTRO DEIONIZATION | ||
|
電気脱イオンは、電気的に活性化した媒体と電位を使用してイオン輸送を発生させて流体からイオン性物質を取り除く水処理プロセスです。このプロセスが、従来からあるイオン交換プロセスなどの他の処理方法と異なる点は、酸や苛性ソーダなどの薬品が不要な事です。これまでのイオン交換装置の場合、不純物が樹脂サイトで取り除かれた後も樹脂による排出機能が継続され、容積の減少が見られました。 |
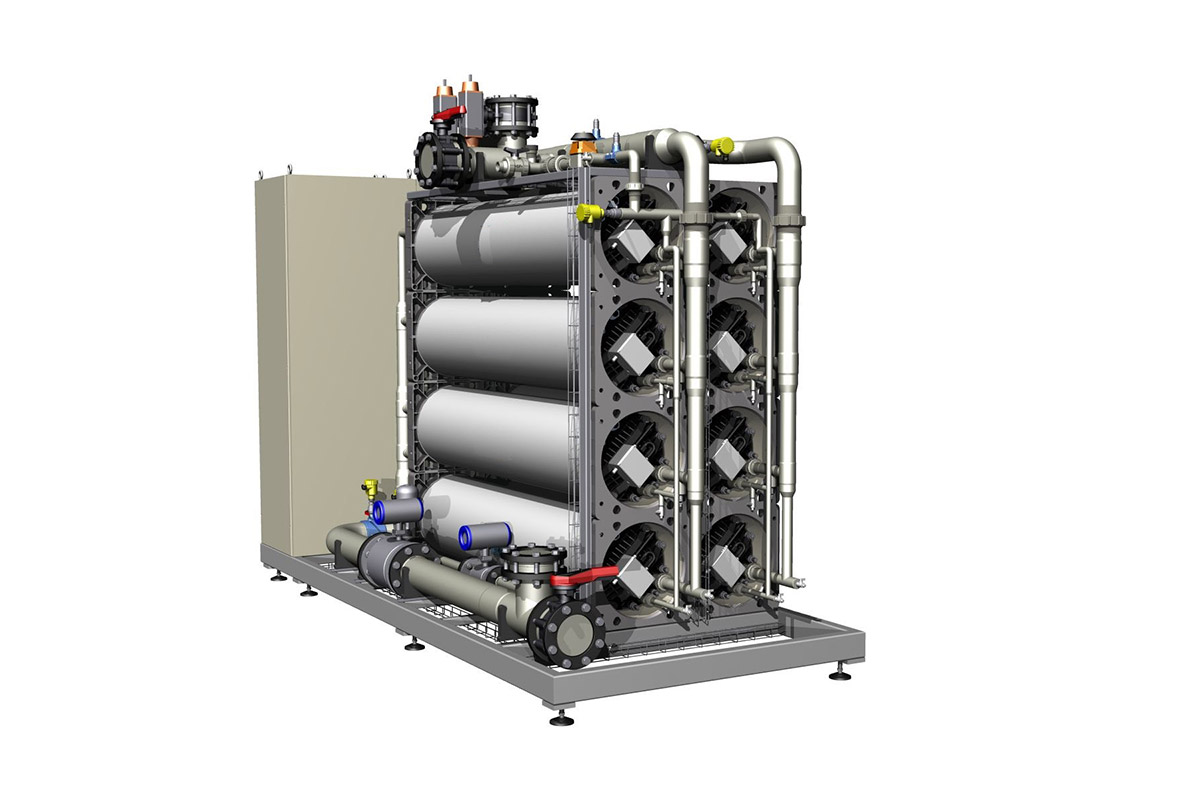 |
Electrodeionization is a water treatment process that removes ionizable species from liquids using electrically active media and an electrical potential to effect ion transport. It differs from other water purification technologies such as conventional ion exchange since it does not require the use of chemicals such as acid and caustic soda. In traditional ion exchange units, after the contaminants are trapped onto the resin sites, the resin continues to exhaust and lose capacity. In electrodeionization the contaminants are continuously removed as they are attracted to one of the two electrical charges and they migrate through the resin bed through ion exchange membranes and into the concentrate stream where they are removed from the device. Electrodeionization is a polishing technology and requires reverse osmosis (RO) as pretreatment. The combination of reverse osmosis – electrodeionization provides the customer with a continuous, chemical-free system. |
|
 |
|||